
In modern toxicology, there has been a significant paradigm shift over the past two decades, moving away from reliance on animal models towards animal-free methods. These approaches, which prioritize in vitro and in silico systems, are increasingly recognized as offering greater relevance for studying human physiology and toxicology.
This shift has been catalyzed by influential documents such as the 2007 publication from the United States titled “Toxicity Testing in the 21st Century: A Vision and a Strategy,” as well as Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes. These publications advocate for the exploration and adoption of alternatives to animal testing.
In 2012, the OECD introduced the concept of Adverse Outcome Pathways (AOPs) framework, which serves as a structured approach to consolidating information about the effects of environmental chemicals or other substances. AOPs delineate the cellular and molecular mechanisms underlying these effects, spanning various levels of biological complexity, including population, individual, organ, tissue, cell, and molecules. By providing a comprehensive framework for understanding toxicological pathways, AOPs facilitate the development of more predictive and efficient testing methodologies, while also reducing reliance on animal experimentation.
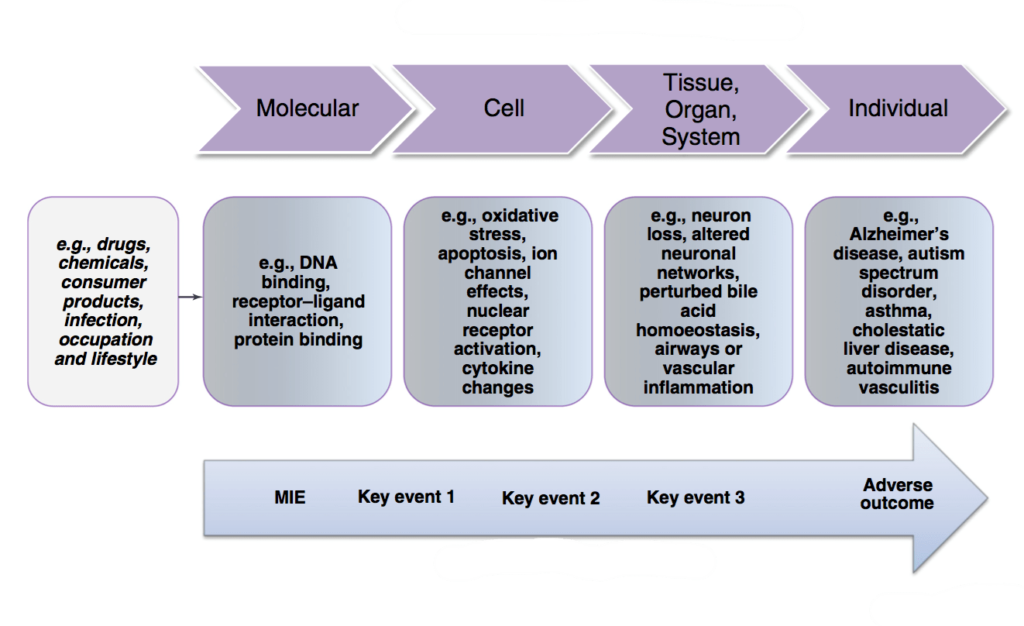
The New Approach Methodologies (NAMs) are all those technologies, methodologies, advanced non-animal approaches that can be used, even in an integrated way, to obtain information on the risk associated with chemicals (3, 4).
The organisation of knowledge about the effects of chemicals through these types of conceptual approaches can be easily applied to the field of biomedical research, for example to identify molecular signals that are altered by the onset of a certain pathology (5, 6).
Examples of initiatives that aim to promote this type of approach in biomedical research are BioMed 21 and Alliance for Human-relevant Science, which bring together scientists and institutions internationally, to overcome the current research paradigm and the adoption/development of new approaches and methodologies, based on the knowledge that traditional models (both in vivo and in vitro) are inadequate.
Bibliography
1.NRC Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press; 2007. 216 p.
2.Directive 2010/63/EU on the protection of animals used for scientific purposes 2010.
3.Afsa [Available from: https://www.afsacollaboration.org/ ]
4.HumamToxomeProject. [Available from: http://human-toxome.com/]
5.Langley GR, Adcock IM, Busquet F, Crofton KM, Csernok E, Giese C, et al. Towards a 21st-century roadmap for biomedical research and drug discovery: consensus report and recommendations. Drug Discov Today. 2017;22(2):327-39.
6.Langley G, Austin CP, Balapure AK, Birnbaum LS, Bucher JR, Fentem J, et al. Lessons from Toxicology: Developing a 21st-Century Paradigm for Medical Research. Environ Health Perspect. 2015;123(11):A268-72.
